
September 5, 2024
Medical Care Cost-free Full-text Medicinal Support For The Treatment Of Weight Problems Existing And Future
Randomized Regulated Test Of Tesomet For Weight Loss In Hypothalamic Obesity European Journal Of Endocrinology At 24 weeks, people had shown no evidence of plateau, which recommended that greater weight management can be accomplished in a year-long trial. This research located that tesofensine generated higher fat burning in overweight rats than in lean Wistar rats. We assumed that this was because of tesofensine's capability to modulate neuronal activity in the LH.Future Viewpoints: Personalized Medication In Weight Problems
Does tesofensine help with weight reduction?
In medical trials, people taking tesofensine experienced considerable fat burning contrasted to those on a sugar pill. Some research studies reported weight-loss of up to 10% of preliminary body weight over a reasonably brief duration.
- Medication development in the area of weight reduction has actually frequently faced pharmacovigilance obstacles, since anorexigenic medications influence numerous neurotransmitter systems and can bring about severe damaging results.
- For CNS drugs being evaluated in weight problems tests, new methods of determining suicidality and various other psychiatric dangers may give not just much more precise security information, however also a better chance at authorization.
- Unfortunately, this research was stopped by the NIH IRB as a result of reasons unrelated to unfavorable medicine effects or efficiency (reinterpretation of the Usual Policy for human subject defense under HHS, 45 CFR 46A).
- Isobolographic analysis was carried out to identify if the communication between 2 drugs given in mix is collaborating (supra-additive), additive, or hostile (infra-additive) [26, 27]
Subjects: Mice
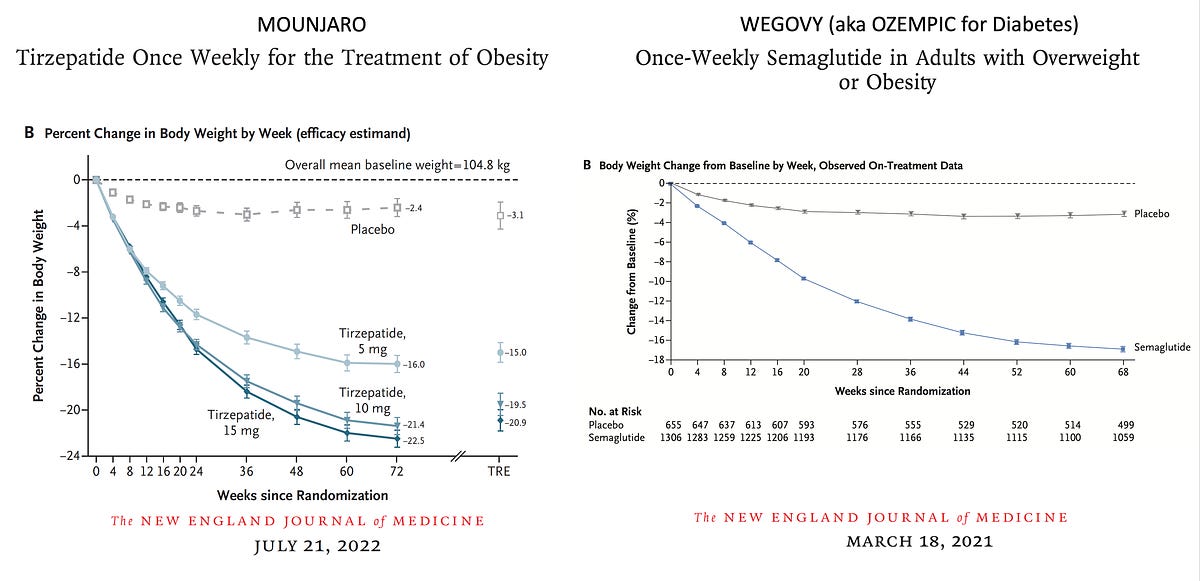
The three-way device of activity, however, might offer serious side-effect problems in large tests. The medicine was launched in 18 EU nations, beginning with the UK in June 2006, under EMEA's conditional authorization. But records of psychiatric side effects restricted its use, excluding clients with significant clinical depression. According to Wolters Kluwer, in May 2008, as adverse-events reports accumulated, the European company updated the tag to show that clinical depression may happen as a side effect in clients without any symptoms aside from excessive weight. After FDA released an approvable letter in February 2006, the agency's advisory board voted 14-0 versus suggesting approval simply 4 months later on, mentioning that Sanofi had stopped working to offer sufficient safety and security data to show that rimonabant's advantages exceeded its threats. " The prospective market for this medication and the continued unpredictability regarding its risks, both known and unidentified, result in our problem about the use of this medication in the general population," FDA team clinical customer Amy Egan told The New York Times.Tesofensine
Craniopharyngioma, the most typical reason for hypothalamic weight problems, has a total incidence of about 1.3-- 1.7 per million people/year (8, 9). Hypothalamic excessive weight establishes in roughly 50% of craniopharyngioma survivors (10, 11). The major negative effects of liraglutide are gastrointestinal signs and symptoms, such as queasiness, diarrhea, irregular bowel movements, and throwing up, and it is advised that the dosage is incrementally enhanced to reduce the incidence of these damaging events. Owing to the postponed gastric draining caused by liraglutide, the action of various other medicines can be impacted. Furthermore, liraglutide usage can create gallstones and, much less typically, acute pancreatitis [57,58]; it needs to not be made use of in people with a history of pancreatitis. Since there are concerns relating to liraglutide use and medullary thyroid cancer cells and numerous endocrine neoplasia, it should not be utilized in patients with a past or family history of such conditions [59-- 61] Orexigen anticipates to file an NDA in the very first half of 2010, according to a company news release. Of main rate of interest is why GLP1R agonism functions so well and how GIP could synergize with GLP1 to improve weight-loss. Short of the results that have actually been achieved in vivo, most especially the 6-month and 1-year professional research studies that appear to show substantial additional benefits of semaglutide when compared to liraglutide, it is hard to refer a molecular basis for that difference. These 2 agents are both extremely powerful and discerning GLP1R agonists, likewise fatty acylated, that provide continual medication plasma concentrations when made use of as prescribed. The difference is not just an issue of prolonged time activity as also a long-action Fc agonist such as dulaglutide does not match the body weight lowering of semaglutide284. 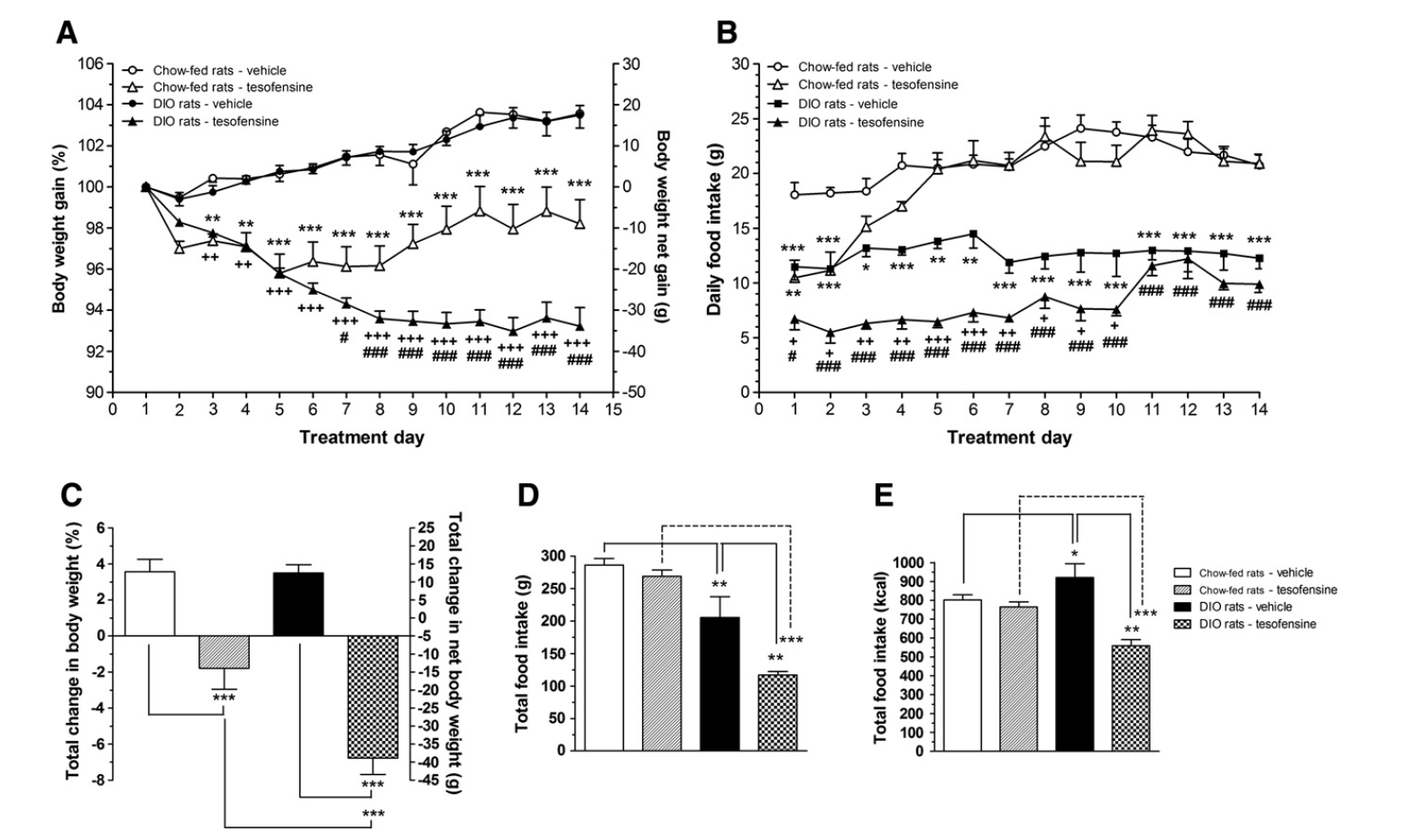
Social Links