
Livingston Nj Pt141 Peptide Therapy For Women Sex-related Wellness
Coverage Of Multi-arm Parallel-group Randomized Tests: Expansion Of The Consort 2010 Statement Guidelines Jama
Nonetheless, when acquired as bremelanotide (Vyleesi), the recommended dose for premenopausal patients with HSDD is "1.75 mg carried out subcutaneously in the abdominal area or thigh, as required, at least 45 minutes prior to any kind of awaited sex" [7] In research studies entailing male individuals, the small sample sizes and brief period of the experiments make it challenging to attract any kind of significant conclusions concerning PT-141's long-lasting safety and security in men. While scientists have noted that PT-141 was "risk-free" [8, 9], this chemical needs to be evaluated in a properly designed https://seoneodev.blob.core.windows.net/pharma-tech/medical-devices/product-management/bremelanotide-subcutaneous-path-adverse.html long-lasting professional test before attracting any final thoughts regarding its safety and security [13]
Getting Ready For The Very First Dosage
- The private investigators have evidence to deny the hypothesis that all the treatments were equally efficient, however no clear indicator of specifically where the distinctions exist.
- Estradiol raises α-MSH degrees in the mediobasal hypothalamus of women rats [110,111], suggesting that α-MSH release may be among numerous intermediaries of estrogen action.
- Therefore, pre-exercise medical clearance is not necessary for asymptomatic individuals getting diabetes treatment consistent with guidelines that desire to start reduced- or moderate-intensity exercise not exceeding the demands of quick walking or day-to-day living.
- Some doctors suggest dosing every 72 hours, or regarding two times a week, permitting more spontaneity in your partnerships.
Cardio Exercise Benefits
The top quality of coverage of multi-arm trials differs considerably, making judgments and interpretation hard. While the majority of the components of the CONSORT 2010 Declaration use just as to multi-arm tests, some elements require adaptation, and, in some cases, additional issues require to be clarified. The ACSM no more includes danger element assessment in the exercise preparticipation health screening procedure.
This exploration caused the exploration of PT-141's special system of activity, identifying it from various other treatments by concentrating on the central nervous system's pathways. This difference is crucial as it emphasizes the peptide's capacity to affect physiological feedbacks in a novel and targeted way. Treatment-emergent adverse events during double-blind therapy (security populace). The solutions given have not been reviewed by the Food and Drug Administration.It is incorrect, as an example, to deduce that the intervention with the most positive results is far better than the others, due to the fact that this inquiry has actually not been examined explicitly. If the major objective is to analyze whether the treatments vary, but not exactly how they vary, it would certainly be proper to compare all groups simultaneously making use of a single international test of value. If the primary goal is to check out a trend, a dose-response model needs to be utilized.
Of the 40% of the individuals taking bremelanotide, just 8.1% ceased the drug during the research as a result of this TEAE [81] There were no clinically substantial modifications to the various other safety measures assessed. Women must start with 1.75 mg subcutaneously 45 mins before sexual activity, like men. Before starting PT-141, females should consult their doctor to figure out the appropriate dose and watch for adverse effects. In 2019, PT-141 was authorized by the United States Food and Drug Administration for the therapy of premenopausal women with hypoactive libido problem (HSDD) [1]
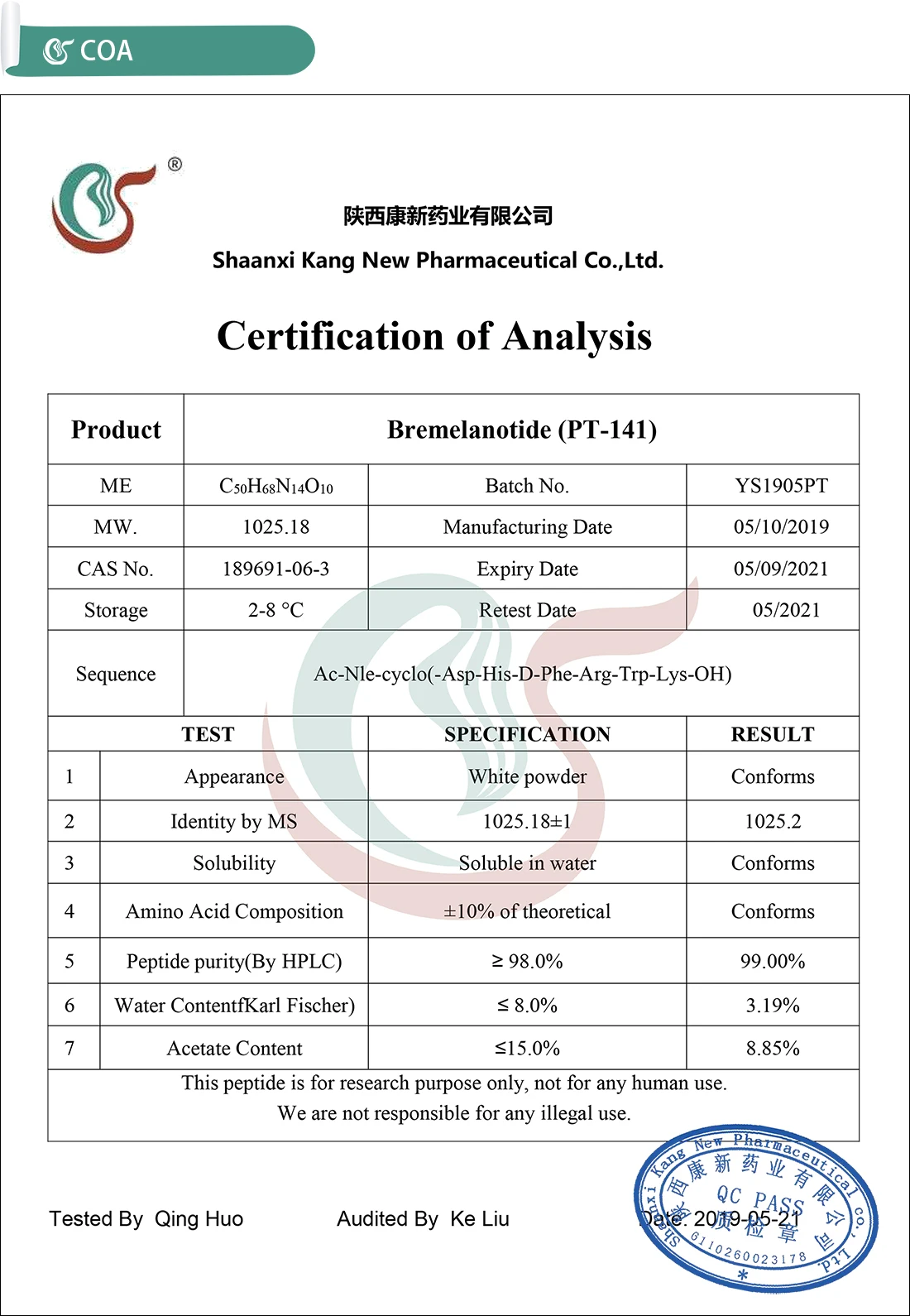
PT-141 is the chemical name for bremelanotide, a melanocortin receptor agonist that was originally established by Palatin Technologies and released under the trademark name "Vyleesi". While no details extension to the basic accompaniment item is recommended below, authors ought to attend to the toughness and constraints of multi-arm tests when it come to problems detailed in the Box. The Table shows the modified list for the coverage of multi-arm parallel-group randomized trials; some products are encompassed cover the coverage requirements connected to the multi-arm style, acknowledging the added complexity imposed by this design. Things that required an expansion from the accompaniment 2010 Statement are explained, with illustratory examples of great coverage. Topics that received bremelanotide experienced more negative adverse effects compared to those who got the placebo (76.6% vs. 58.2%). In both research studies, bremelanotide individuals experienced more regular nausea (40% vs 1.3%), flushing (20.3% vs 0.3%), and headache (11.3% vs 1.9%) than the sugar pill team. As mirrored in the original phase, 2b study, mean increases in subject BP and HR were moderate.