
September 5, 2024
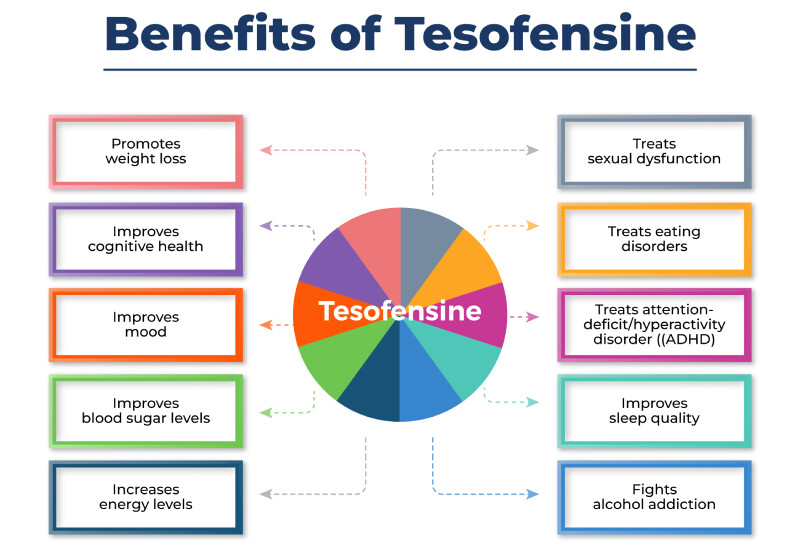
How Tesofensine Encourages Weight Reduction
- From randomization to year one, subjects provided the 3.0 mg dose of liraglutide lost 5.8 kg more weight thanplacebo and at year 2 weight reduction was 3.0 kg in excess of sugar pill [90]
- PYY3-36 has high fondness for the NPY receptor Y2, which is just one of a number of NPY receptors that play vital duties in the guideline of food intake.
- Provided the capacity of tesofensine to regulate the activity of the LH, our preclinical searchings for agree with the proposal that tesofensine can be a beneficial therapy for people with hypothalamic weight problems, a rare feeding problem, as lately demonstrated [38]
- One prominent example below is rimonabant, an endocannabinoid 1 receptor (CB1) antagonist shown to decrease appetite, enhance thermogenesis and lessen lipogenesis preclinically and in various human trials333.
Related Terms:
The triple system of activity, however, might present serious side-effect issues in large-scale tests. The medication was introduced in 18 EU countries, starting with the UK in June 2006, under EMEA's conditional approval. But records of psychological adverse effects restricted its usage, leaving out people with major anxiety. According to Wolters Kluwer, in Might 2008, as adverse-events records piled up, the European company updated the tag to suggest that depression may occur as an adverse effects in people with no signs besides obesity.Which of the adhering to is an effective therapy for excessive weight?
The Prospective Impact On Weight Problems
It was postulated that although 5-HT1A agonists were not appropriate for advancement as novel antihypertensive medicines, they may be adequately effective to stop the boosts in blood stress and heart price click here created by sibutramine (Heal and Cheetham, 2001). This concept was verified by showing that sibutramine-induced rises in blood pressure and heart rate in mindful, telemetered rats were abolished by co-administration of the selective 5-HT1A agonist, flesinoxan. These searchings for formed the basis for a patent declaring on this medicinal mix (Heal and Cheetham, 2001). Prosidion also established PSN-1 and PSN-2, which incorporated potent noradrenaline reuptake inhibition and 5-HT1A agonism in the same molecule (Thomas et al., 2006). These substances lowered food intake and generated weight-loss in both DIO female (Fig. 2) and high fat-fed male overweight rats (Thomas et al., 2006). Additionally, Tesofensine obesity research study reveals that this sort of medication reduces hunger and advertises volume, enabling individuals to consume less without really feeling denied. Therefore, those that used Tesofensine for obesity felt much less cravings, which aided them endure their calorie deficiency for longer periods of time. This might be especially beneficial for people who go to risk of establishing kind 2 diabetes as a result of obesity-related insulin resistance. Tesofensine obesity dealing with deals a promising service for those dealing with obesity since its effects are both rapid and durable when taken constantly over prolonged periods of time. If approved by the FDA it could provide a much required alternative for those aiming to take control of their weight and boost their total wellness and wellness via successful weight management management. These pilot results be entitled to even more expedition to far better assess the benefit-risk ratio of tesofensine in the treatment of PD. A greater proportion of individuals reacted with a minimum of 20% (variety, 26% -40%) enhancement in UPDRS subscale II plus subscale III overall score in all the tesofensine arms of the trial compared with placebo (14%) (Table 3). A higher percentage of patients reacted with a minimum of 20% enhancement in off time in the 3 highest-dosage tesofensine treatment groups than in the sugar pill team.Social Links