September 5, 2024
Tesofensine, An Unique Antiobesity Medication, Silences Gabaergic Hypothalamic Neurons Pmc

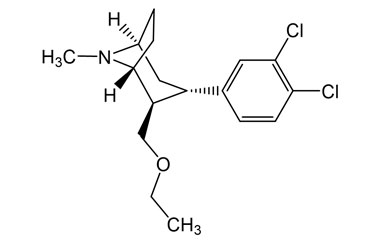
Health Care Free Full-text Medicinal Assistance For The Treatment Of Weight Problems Existing And Future One of the most usual problems in patients treated with subcutaneous liraglutide 1.8 mg are gastrointestinal adverse effects including nausea or vomiting, diarrhea, vomiting and constipation77. The much more recently FDA-approved semaglutide at a dosage of 2.4 mg lowers imply body weight to ~ 15% after 68 weeks of therapy (relative to ~ 2.4% in sugar pill controls) 38. The medicine is typically well tolerated although the normal GLP1-related unfavorable impacts (mostly nausea or vomiting, diarrhea, throwing up and irregularity) still prevail38. Tesofensine (( 1R, 2R, TWO, FIVE) -3-( 3, 4-dichlorophenyl) -2-( ethoxymethyl) -8- methyl-8-azabicyclo [3.2.1] octane)) is a novel potent, non-selective uptake inhibitor of NE, DA and 5-HT (Astrup et al., 2008b).
Is Tirzepatide Far Better Than Semaglutide?
- Bupropion is structurally comparable to the cravings prevention diethylpropion [98, 99] and can block presynaptic reuptake of both norepinephrine and dopamine, generally known as antidepressants.
- GLP-1R agonists potentiate glucose-induced insulin secretion (GIIS) from pancreatic β-cells, which potently boosts insulin secretion and improves insulin sensitivity in adipose tissue, via enhanced β-cell activity of GIPR.
- Eventually, only in human research study can the assessment of whether GDF15 analogues will verify effective and safe for weight loss administration be determined267.
- NB-32 SR (Contrave) was accepted for the treatment of weight problems in 2014and brings the black box warning concerning suicidal ideation and actions typical ofanti-depressant medications.
- Various other just recently created GLP-1 agonists with prolonged half-lives such as taspoglutide and albiglutide may also allow weekly application.
Associated Terms:
The very first research of youngsters given 2 mg exenatide once a week for a 12-month period again revealed no considerable effect on weight or BMI, albeit one individual showed a BMI SDS reduction of -0.33 after one year (109 ). On the other hand, a current randomized, multicentre, double-blind, placebo-controlled trial was carried out in 10- to 25-year-olds with hypothalamic injury adhering to intracranial tumor and hypothalamic weight problems. Participants were randomised to once-weekly subcutaneous injections of exenatide 2 mg or placebo for 36 weeks. Exanetide was normally well endured with most of side effects being connected to stomach disturbance (110 ). In addition, a pick team of individuals with limited hypothalamic damages might respond better to GLP1A, whilst others with more substantial hypothalamic damage fall short to react to the same treatment. The writers guessed that interruption of hypothalamic pathways associated with hunger and energy homeostasis may cause modifications in various other pathways such as GLP1-mediated signalling in the brainstem, which remain intact in people with hypothalamic obesity (111 ).What class of drug is tesofensine?
Tesofensine is a Serotonin-norepinephrine-dopamine-reuptake-inhibitor (SNDRI). SNDRIs are a class of psychedelic antidepressants. They act upon neurotransmitters in the mind, specifically, serotonin, norepinephrine and dopamine.
Glp-1 Physiology In Excessive Weight And Development Of Incretin-based Medications For Chronic Weight Management
Numbers 1 and 2 sum up the major device of activity for current anti-obesity drugs used to treat weight problems (Table 1). As a matter of fact, regarding 70% of individuals who are overweight have less dopamine receptors than patients of regular weight. The market for weight-reducing medications has had a somewhat chequered background, qualified by major product withdrawals due to safety worries. The comparative effectiveness of liraglutide was examined over and listed below aBMI of 35kg/m2 and found that liraglutide carried out just as well inboth classes of obesity [99] Efficiency ofliraglutide was contrasted throughout racial groups and was shown to offer similarweight loss [100] The pooled range data was also used to evaluateearly weight loss as a forecaster for responders.Social Links