September 5, 2024
The Capacity Of Tesofensine: Browsing Via A Reliable Cycle For Weight Administration
The Capacity Of Tesofensine: Browsing Through A Reliable Cycle For Weight Management In both groups, better weight-loss after 3 months of therapy was a forecaster of a great reaction condition. Although diet plan and workout are the key treatments for obesity, these activities are frequently supplemented making use of appetite suppressants. Tesofensine (NS2330) is a three-way monoamine re-uptake prevention with an affinity for dopamine (DAT), serotonin (SERT), and norepinephrine (INTERNET) transporters.
- Unfavorable events in the safety and security populace of a randomised clinical trial of Tesomet for hypopituitary individuals with hypothalamic excessive weight by System Organ Course and Preferred Medical Term.
- The distance of each nerve cell to the centroid of their respective cluster was then calculated.
- Identifying if weight management is long-term needs long-lasting maintenance of healthy and balanced habits and way of life adjustments.
- Medical solution specialists will commonly prescribe this medicine for a details period.
Can You Integrate Tesofensine And Semaglutide?
However, it's important to note that private reactions can differ, and adverse effects might occur, such as nausea or vomiting, intestinal pain, dry mouth, or modifications in bowel movements. Temporary impacts must be kept track of very closely, and it's vital to comply with the suggested dosage and guidelines supplied by a healthcare expert. However, whereas weight-loss impacts generally translate from rats to humans, maximal efficiency is traditionally 2 to 4 times lower in human beings about rodents (Fig. 3). It can be said that higher family member weight loss in rats is expected as mice have a higher mass-specific energy expense than humans, with a greater contribution of brown adipose tissue to metabolic rate128. As a result, computer mice may be a lot more prone to drugs that affect energy expenditure. The high mass-specific metabolic rate requires completely high calorie intake to shield against a chronic deficit in power equilibrium. Posner's group considered a total of 1,201 "individual stories" from 7 rimonabant trials. Making use of C-CASA, they classified 91 cases as either "potentially" or "absolutely" self-destructive, yet removed some due to the fact that they occurred in study arms without sugar pill control. The final tally of suicidality instances was 74, with 20 on sugar pill, 8 on rimonabant 5 mg, and 46 on rimonabant 20 mg; the overall drug-to-placebo ratio was 1.8 to 1.System And Therapies Of Antipsychotic-induced Weight Gain
Making use of antipsychotics in children and teenagers has increased significantly over the past few years. It is disconcerting that kids and teenagers get more weight than their grown-up equivalents, which predisposes them to consequences of weight gain for several years. Weight gain in youngsters has negative physical health and wellness along with mental wellness repercussions. Nevertheless, the weight reduction attained with a 0.5 mg dosage (9.2%) was only a little less than that of a 1 mg dose (10.6%). Thinking about the dose-dependent rise in adverse effects, it questions regarding the justifiability of greater doses. Once the first therapy phase is full, individuals that have successfully reached their weight loss objectives can change to a maintenance stage of taking this medication. In this period, they may take smaller doses as a method to avoid regaining weight and receive their progression in shedding it. Prior to starting a Tesofensine routine, you need to speak to a qualified medical service expert. An all-compassing clinical evaluation has to be carried out to figure out eligibility for the medication's use by thinking about health and wellness history, present medications being taken, and feasible incongruities.What are the threats of taking tesofensine?
Tesofensine 0.25 mg, 0.5 mg, and 1.0 mg and diet plan induced a mean weight reduction of 4.5% (0.87 ), 9.2% (0.91 ), and 10.6% (0.84 ), specifically, higher than diet and sugar pill (p<< 0.0001). One of the most common damaging occasions caused by tesofensine were dry mouth, queasiness, bowel irregularity, difficult feceses, diarrhea, and sleeplessness.
Tesofensine Minimized Feeding Habits Caused By Optogenetic Activation Of Lh Gabaergic Nerve Cells In Lean Vgat-chr2 Mice
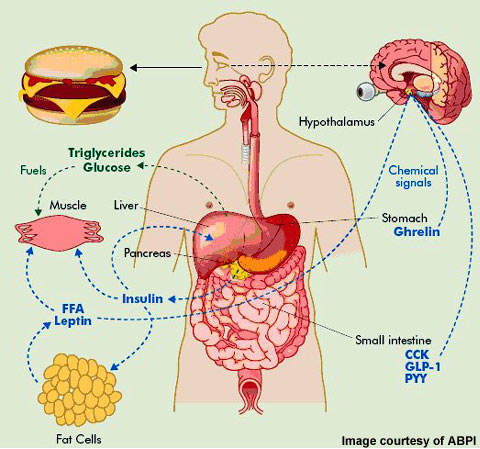
In feedback to the food consumption, it boosts the sensation of satiation and prevents cravings in the hypothalamic facilities by activating the NPY2R receptor (neuropeptide Y receptor kind 2) [211,212] It was likewise located to slow down gastric emptying, inhibit the secretion of tummy acid, exocrine pancreatic function, and insulin [213] Research studies in pet versions validate the efficiency of PYY analogues, both in weight and food consumption decrease [214,215] Researches on people conducted to date do not enable a clear assessment of the efficiency of PYY in weight decrease yet. In a 12-week, randomized study, intranasal administration of 200 or 600 μg of PYY 3 times a day prior to each dish in overweight people (BMI 30-- 43 kg/m2), no significant weight reduction demonstrated [216] It enhances the focus of dopamine and norepinephrine in the areas of the mind in charge of inspiration, reward, focus, and impulsivity. Moreover, it is known that tesofensine activates α1 adrenergic receptors and, to a minimal level, dopamine D1 receptors [2-- 4] It exhibits powerful antiobesity effects, yet the underlying mobile devices are still being proactively explored. This research initially intends to determine the neuronal correlates of tesofensine-induced weight reduction in the Lateral Hypothalamus (LH) in lean and overweight rats. Involvement of GIPR agonism for the treatment of obesity and T2D is regarded with remarkable scepticism, as the insulinotropic impact of GIP is diminished in people with T2D179. Weight-loss medicines might be considered when other approaches have actually not led to enough weight reduction or when there is a demand to resolve weight-related health issues. It is necessary to note that the decision to take weight-loss medications ought to be made in assessment with a healthcare professional. Of primary rate of interest is why GLP1R agonism functions so well and exactly how GIP might synergize with GLP1 to improve weight reduction. These two agents are both extremely powerful and discerning GLP1R agonists, similarly fatty acylated, that supply continual drug plasma focus when made use of as suggested.Social Links