September 5, 2024
Comprehensive Testimonial Of Present And Upcoming Anti-obesity Medicines Pmc
Clinical Weight-loss Fleming Island, Fl We used a chi-square test to analyze distinctions in the proportion of nerve cells hired. For subcutaneous catheter implantation, the rats went through two little incisions (∼ 1mm) in the remarkable left abdomen and dorsal neck locations. Sterilized silicone tubes (12 centimeters long, Silastic laboratory tubes, Dow Corning, Midland, MI, PET CAT. No. 508-- 004) was made use of as a catheter and tunneled subcutaneously from the back cut to the dorsal neck incision. After surgery, the rats were treated with intraperitoneal enrofloxacin (10 mg/kg) and meloxicam (2 mg/kg) for 3 consecutive days. The electrophysiological information was collected and refined as described in extracellular recordings in mice.Impacts Of Anti-obesity Drugs On Eating Behavior And Neural Activity
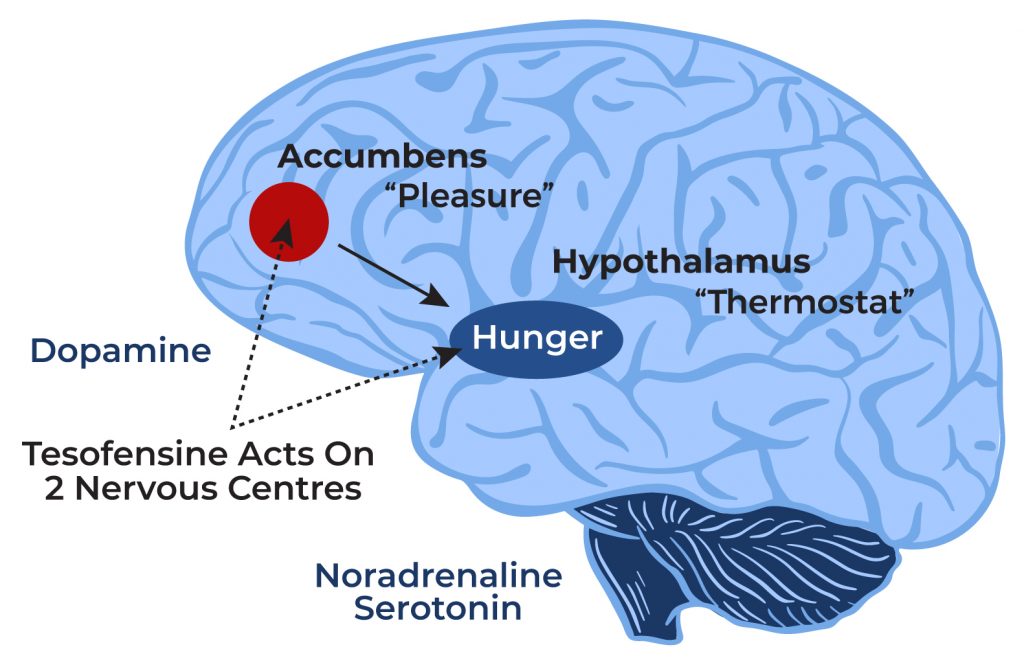
Patient demographics, baseline disease attributes, and concomitant PD treatment are given up Table 1. Arise from a scientific trial revealed that weight management with tesofensine peptide was significantly higher over a six-month duration than those accomplished with any of the drugs presently readily available. Weight management depended on 10.6% in patients, which was about two times the weight loss generated by medicines currently accepted by the US FDA for dealing with obesity. The results of the trial, published in The Lancet, reveal that all dosages of tesofensine generated a considerably better mean weight reduction than sugar pill and diet. For instance, patients getting the 0.5 mg dosage revealed a 9.2% mean weight decrease (representing 9.1 kg) above that of placebo, and the percentage of clients that accomplished greater than 5 kg or even more fat burning was 87%, compared with 29% in the sugar pill team. The 2nd bigger group of cells that were extra highly regulated by tesofensine in obese than in lean rats was the set of nerve cells displaying a durable inhibition (see E1 in Fig 2). Other weight-loss medications and medicine combinations are in the growth process, including bupropion/naltrexone, bupropion/zonisamide, and tesofensine. The FDA approved Qsymia ™ (Vivus, Inc., Mountain View, The Golden State, USA), a combination of phentermine and topiramate extended-release (PHN/TPM), most recently. Tesofensine is being hailed as a game-changer in the weight reduction industry because of the significant results received scientific trials. The medication has been located to be effective in weight-loss, better insulin level of sensitivity and, when incorporated with exercise and appropriate diet, can result in substantial and resilient weight loss. In the secondary endpoint evaluation of all professional tests, the phentermine/topiramate CR team showed substantial renovations in cardiometabolic danger factors, including waistline area, glycemic control, and lipid account [37,38] Naltrexone ER/bupropion emergency room likewise caused substantially higher glycosylated hemoglobin (HbA1c) decrease (− 0.6% vs. − 0.1%) than the sugar pill [31] The device of action and application schedule of anti-obesity medications are summed up in Fig. The long-term impacts of 4 approved medicines on weight reduction, cardiometabolic criteria, and safety and security profiles are summed up in Table 1, Fig. The suggested formula for the monitoring of weight problems with offered long-lasting anti-obesity medicines is summed up in Fig. Hypothalamic excessive weight is a tough problem to treat, as there are currently no accepted or efficient medicinal treatments. However, tesofensine is a novel compound with potential in human research studies and might be a promising alternative for these clients [38] Provided the capacity of tesofensine to regulate the task of the LH, our preclinical findings concur with the proposition that tesofensine can be a beneficial therapy for individuals with hypothalamic weight problems, an uncommon feeding problem, as lately demonstrated [38] Tesofensine is an unique triple monoamine reuptake inhibitor that is currently being examined for the therapy of obesity. It prevents the reuptake of the neurotransmitters serotonin, norepinephrine, and dopamine, resulting in increased degrees of these monoamines in the synaptic cleft. Tesofensine was initially developed for the therapy of Alzheimer's disease and Parkinson's illness, but was found to induce weight-loss during clinical tests. This triggered even more study into its potential as an anti-obesity medication.Tesofensine has actually demonstrated promising fat burning effects in phase II and III medical tests. Research studies have actually shown that tesofensine can generate dose-dependent fat burning of approximately 10% of first body weight over 6 months of treatment. This weight-loss is higher than what is generally seen with other authorized anti-obesity medications.Statistical Analysis
Several side effects of topiramate belong to the restraint of carbonic anhydrase activity, consisting of metabolic acidosis, hypokalemia, kidney stones, angle-closure Click here glaucoma, nearsightedness, and anhidrosis. Associated signs should be observed carefully and the drug needs to be terminated as soon as signs and symptoms occur. Notably, topiramate ought to not be integrated with various other drugs that inhibit carbonic anhydrase.- This timing can be useful as it enables the medicine to take effect when you may require the most sustain in handling your hunger.
- But, unless you have an unfavorable reaction, you'll feel happier, much more stimulated, assume a little faster, and most importantly, you'll eat less, move a lot more, and burn even more of the calories you require in order to slim down fast.
- This might be because POMC, which is self-inhibited by endogenous opioids, can lower the appetite-suppressing impacts of bupropion.
- The purpose of today research was consequently to establish whether persistent treatment with psilocybin would apply effects on weight gain in an animal design of weight problems.
How much weight can you shed on tesofensine?
For those taking the lowest dosage of 0.25 mg, average weight reduction was 6.5%, those taking the tool dose of 0.5% lost 11.2% and those taking the highest dosage of 1 mg lost 12.6%. In both highest dose groups, the treatment brought about a 4 factor decrease in BMI in a period of 24 weeks.

Social Links