
August 16, 2024
Secure Gastric Pentadecapeptide Bpc 157 Therapy For Main Abdominal Compartment Syndrome In Rats
Stomach Pentadecapeptide Bpc 157 As A Reliable Treatment For Muscular Tissue Crush Injury In The Rat Surgery Today Yes, BPC-157 has revealed promise in helping the healing of joint and muscular tissue injuries. It can help fix damages to ligaments, tendons, and muscular tissues, promoting faster healing and decreasing the danger of problems. At Amazing Medications, our doctors regularly recommend the top-notch PDA peptide to people after an evaluation and individualized therapy plan.BPC-157 and TB-500: Inflammation, Tissue Damage, and More - The Portugal News
BPC-157 and TB-500: Inflammation, Tissue Damage, and More.
Posted: Tue, 19 Sep 2023 07:00:00 GMT [source]

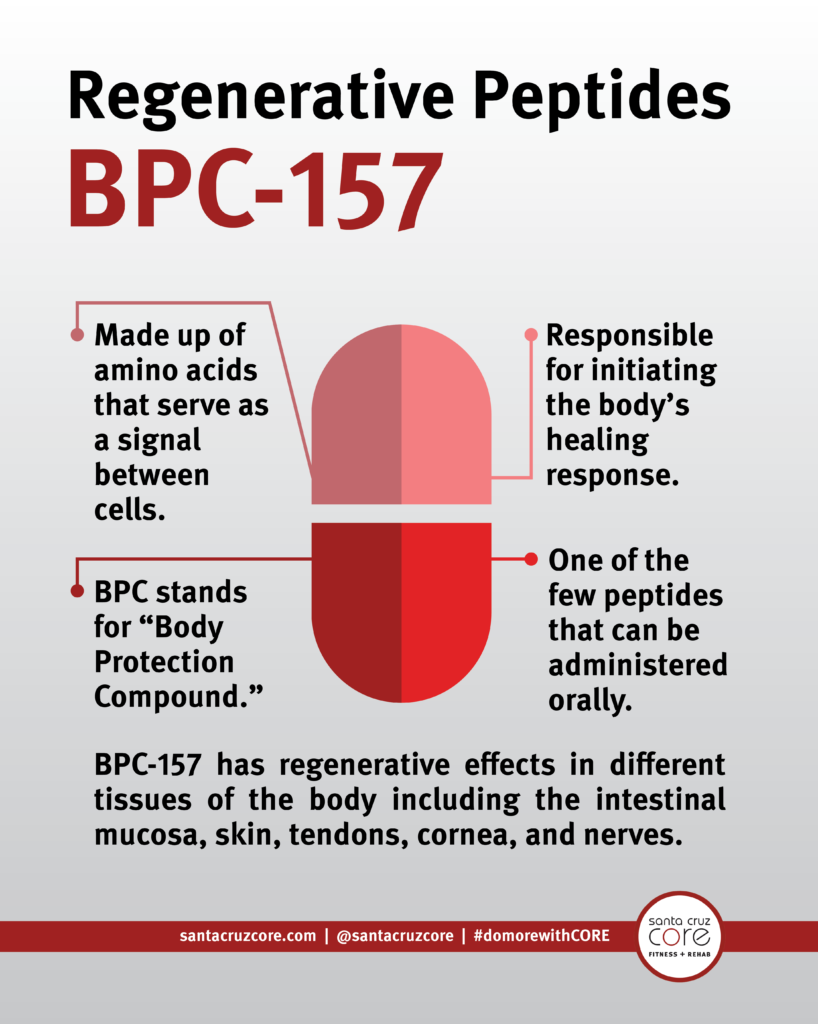
Just How To Mix Bpc 157
- In the bodybuilding/fitness world, BPC-157 is typically utilized to sustain recovery from training-related injuries and improve total performance.
- A previous study35 has actually shown that BPC-157 cream enhances healing of shed injuries brought on by direct exposure to route flame.
- Utilizing Masson discoloration, we located that the degree of collagen deposition was considerably higher in BPC-157- and bFGF-treated groups.
- The landscape of neuroprotection too discovers a brand-new engineer in BPC-157, safeguarding neuronal honesty versus the persistent attack of degenerative forces.
- In the liver and kidney, just moderate blockage was observed at the highest intra-abdominal pressures.
Examining Research Study End Results For Different Kinds Of Administration
The research study right into BPC-157's anti-tumor effects is still in the initial phases, with a lot of studies performed artificial insemination (cell cultures) or in pet models. While these research studies recommend that BPC-157 might have anti-tumor homes, more comprehensive study, consisting of clinical tests, is essential to totally recognize its prospective and devices of action in cancer cells treatment. While promising, the research on the emotional effects of BPC-157 is still in the preliminary stages, mainly based on pet designs.Medical Assessments
Consequently, in rats with esophagogastric anastomosis that were treated with L-NAME, the degree of sphincter failing was greater, in accordance with the most awful esophageal and stomach lesions, and increased lethal outcomes. One group of individuals who can potentially take advantage of utilizing BPC 157 are those that suffer from stomach concerns. BPC 157 has been shown to promote intestinal healing, which might be useful for individuals with conditions like Crohn's condition, ulcerative colitis, and short-tempered bowel disorder. Furthermore, BPC 157 has actually been shown to lower swelling in the intestine, which can aid to ease signs and symptoms in people with these conditions. Exploring the record of clinical examination, the genesis of BPC-157 was a result that pivoted on experimental studies very closely aligned with gastrointestinal system research study. No brand-new metabolites were discovered in pee, bile, and fecal examples besides the six components discovered in the plasma. In the combined pee examples accumulated from 0 to 8 h, the content of [3H] proline (M1), the main metabolite, was higher, accounting for 13.9% (lady) and 11.7% (man) of the total radioactivity. In mixed pee examples gathered in between 8 and 72 h, the proportion of tritium water was greater, making up 69.5% (female) and 75.3% (man) of the overall radioactivity, and [3H] proline (M1) represented 3.11% (woman) and 4.17% (male) of the complete radioactivity (Figure 5B). The complete radioactivity discharging in mixed bile examples collected in between 0 and 72 h was reduced, and tritium water was mainly found, making up 91.2% (ladies) and 91.0% (men) of the sample. The rats were euthanized, and cells samples (mind, heart, kidneys, liver, spleen, lung, stomach, intestine, muscle mass, oil, ovaries, womb, testicles, and thymus) were accumulated at 3 minutes, 10 min, 1 h, and 24 h after administration (three men and 3 females at each time factor). Male SD rats were provided a single IM shot of blank solvent (excipient), and organic examples, consisting of entire blood, plasma, pee, feces, and cells, were collected for background control. The radioactivity of the plasma, tissue, bile, urinary, and fecal examples was analyzed making use of a fluid scintillation counter. An overall of 324 SD rats were arbitrarily split right into five groups, consisting of 66 rats in group one, 60 rats each in teams two to four, and 78 rats in group five, with each team comprising fifty percent male and half female topics. Teams two, three, and 4 were provided 20, 100, and 500 μg/ kg BPC157 saline options through single IM injections, specifically. https://s3.us-east-1.wasabisys.com/2udlbbfu4jfp72izc/medication-safety/pharmacology/bpc-157-peptide-therapy-bpc-157-pure-advantages-bpc-157.html
Does BPC 157 boost muscular tissue development?
Extra capillary indicate raised blood flow, nutrient supply, and removal of waste products from muscle cells, all of which are beneficial for bodybuilding. That said, it''s crucial to keep in mind that while BPC 157 does promote muscular tissue development, its primary role is in healing and decreasing swelling.
Social Links